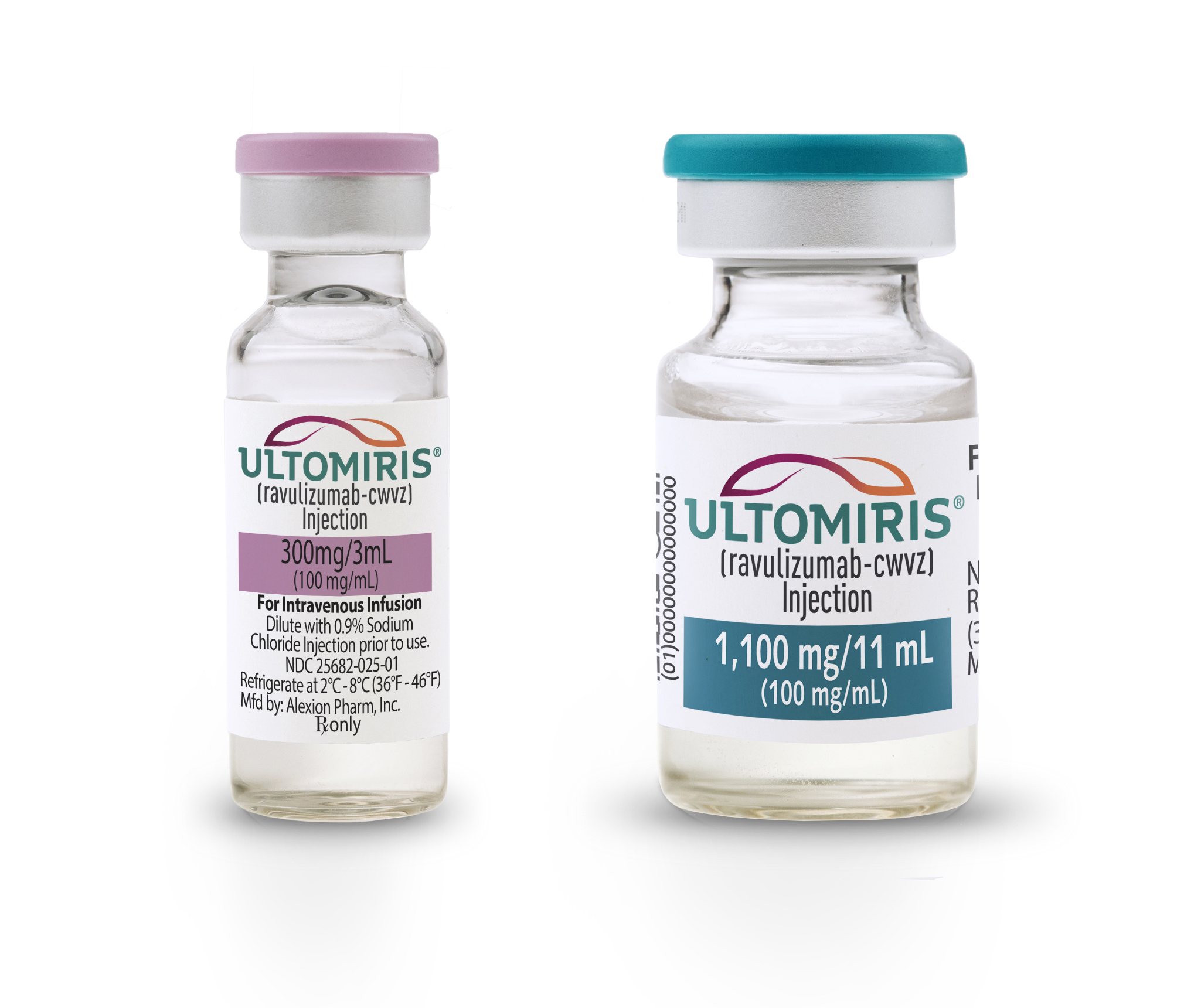
Each single-dose vial contains 300 mg ravulizumab-cwvz at a concentration of 10 mgmL with a pH of 70. ULTOMIRIS ravulizumab-cwvz injection 10 mgmL is a sterile clear to translucent slight whitish color preservative-free solution for intravenous use.
 Alexion Receives Fda Approval For New Advanced Formulation Of Ultomiris Ravulizumab Cwvz With Significantly Reduced Infusion Time Business Wire
Alexion Receives Fda Approval For New Advanced Formulation Of Ultomiris Ravulizumab Cwvz With Significantly Reduced Infusion Time Business Wire
1 billable unit 10 mg NDC.

Injection ravulizumab cwvz 10 mg. J1303 is a valid 2021 HCPCS code for Injection ravulizumab-cwvz 10 mg or just Inj ravulizumab-cwvz 10 mg for short used in Medical care. 21 2018 the North Carolina Medicaid and NC Health Choice programs cover ravulizumab-cwvz injection for intravenous use Ultomiris for use in the Physician Administered Drug Program when billed with HCPCS code J3590 - Unclassified Biologics. Subscribe to Codify and get the code details in a flash.
ULTOMIRIS ravulizumab-cwvz injection 10 mgmL is translucent slight whitish color solution supplied in single-dose vials as. ULTOMIRIS ravulizumab-cwvz injection 10 mgmL is translucent slight whitish color solution supplied in single-dose vials as. Ultomiris 300 mg3 mL single-use vials for injection.
ULTOMIRIS ravulizumab-cwvz injection for intravenous use Initial US. Ravulizumab-cwvz for the treatment of adult patients with paroxysmal nocturnal hemoglobinuria PNH. Effective with date of service Dec.
25682-0022-xx Ultomiris 1100 mg11 mL single-use vials for injection. Injection ravulizumab-cwvz 10 mg Temporary Codes for Use with Outpatient Prospective Payment System C9052 is a valid 2021 HCPCS code for Injection ravulizumab-cwvz 10 mg or just Injection ravulizumab-cwv for short used in Other medical items or services. 300 mg30 mL 10 mgmL carton containing one vial.
25682-0025-xx Ultomiris 300 mg30 mL single-use vials for injection. C9052 HCPCS Deleted Code for Injection ravulizumab-cwvz 10 mg C9052 Deleted code effective Oct. 1 2019 Subscribe to Codify and get the code details in a flash.
Drugs administered other than oral method chemotherapy drugs. Life-threatening meningococcal infectionssepsis have occurred in patients treated with ULTOMIRIS and may become rapidly life-. See full prescribing information for complete boxed warning.
Injection ravulizumab-cwvz 10 mg. This increases your chance of getting very serious possibly fatal infections especially meningitis or sepsis. PNH is a rare acquired disorder that leads to the rupture or destruction of red blood cells.
J1303 Injection ravulizumab-cwvz 10 mg. Ravulizumab-cwvz injection is used in adults to treat paroxysmal nocturnal hemoglobinuria PNH. A type of anemia in which too many red blood cells are broken down in the body so there are not enough healthy cells to bring oxygen to all parts of the body.
Store ULTOMIRIS vials refrigerated at 2C -8C 36F -46F in the original carton to protect from light. SERIOUS MENINGOCOCCAL INFECTIONS. HCPCS Code for Injection ravulizumab-cwvz 10 mg J1303 HCPCS code J1303 for Injection ravulizumab-cwvz 10 mg as maintained by CMS falls under Drugs Administered by Injection.
Get medical help right away if you develop symptoms such as nauseavomiting that doesnt stop high fever chills severe headache stiff neck mentalmood changes such as confusion eye sensitivity to light. Ravulizumab-cwvz injection for intravenous use Initial US. J1303 Effective 10119 Injection ravulizumab-cwvz 10 mg C9052 Injection ravulizumab-cwvz 10 mg Applicable NDCs 25682-0022-xx Ravulizumab-cwvz Ultomiris 300 mg30 mL Intravenous Solution Applicable Diagnosis Codes ICD-10 ICD-10 Description D595 Paroxysmal nocturnal hemoglobinuria Marchiafava-Micheli Revision History.
300 mg30 mL 10 mgmL carton containing one vial. Ultomiris will be available as a 300 mg30 mL single dose vial for injection. Ravulizumab-cwvz can lower your bodys ability to fight infections.
 Ultomiris For Paroxysmal Nocturnal Haemoglobinuria Usa
Ultomiris For Paroxysmal Nocturnal Haemoglobinuria Usa
 Fda Approves New Formulation Of Ultomiris For Pnh And Ahus
Fda Approves New Formulation Of Ultomiris For Pnh And Ahus
 Ultomiris For Paroxysmal Nocturnal Haemoglobinuria Usa
Ultomiris For Paroxysmal Nocturnal Haemoglobinuria Usa
 Ultomiris Ravulizumab Cwvz Ordering
Ultomiris Ravulizumab Cwvz Ordering
 Ultomiris Intravenous Uses Side Effects Interactions Pictures Warnings Dosing Webmd
Ultomiris Intravenous Uses Side Effects Interactions Pictures Warnings Dosing Webmd
 Ultomiris Ravulizumab Dosing Indications Interactions Adverse Effects And More
Ultomiris Ravulizumab Dosing Indications Interactions Adverse Effects And More
 Ravulizumab Injection For Blood Disorder Future Medicine India
Ravulizumab Injection For Blood Disorder Future Medicine India
 Alexion Receives Early Fda Approval For Ultomiris Ravulizumab Cwvz In Adults With Paroxysmal Nocturnal Hemoglobinuria Pnh Business Wire
Alexion Receives Early Fda Approval For Ultomiris Ravulizumab Cwvz In Adults With Paroxysmal Nocturnal Hemoglobinuria Pnh Business Wire
 Alexion Receives Fda Approval For New Advanced Formulation Of Ultomiris Ravulizumab Cwvz With Significantly Reduced Infusion Time Business Wire
Alexion Receives Fda Approval For New Advanced Formulation Of Ultomiris Ravulizumab Cwvz With Significantly Reduced Infusion Time Business Wire
Https Www Ultomiris Com Pdf Ultomiris Pharmacy Admin Guide Pdf
 Ultomiris For Paroxysmal Nocturnal Haemoglobinuria Usa
Ultomiris For Paroxysmal Nocturnal Haemoglobinuria Usa
 Alexion Receives Early Fda Approval For Ultomiris Ravulizumab Cwvz In Adults With Paroxysmal Nocturnal Hemoglobinuria Pnh Alexion Pharmaceuticals Inc
Alexion Receives Early Fda Approval For Ultomiris Ravulizumab Cwvz In Adults With Paroxysmal Nocturnal Hemoglobinuria Pnh Alexion Pharmaceuticals Inc
 Ultomiris Ravulizumab Cwvz Dosing
Ultomiris Ravulizumab Cwvz Dosing
 Alexion Receives Early Fda Approval For Ultomiris Ravulizumab Cwvz In Adults With Paroxysmal Nocturnal Hemoglobinuria Pnh Business Wire
Alexion Receives Early Fda Approval For Ultomiris Ravulizumab Cwvz In Adults With Paroxysmal Nocturnal Hemoglobinuria Pnh Business Wire
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.