Each single-dose prefilled syringe for subcutaneous use contains 100 mg of guselkumab in 1 mL. NAPA Auto Parts Clarksville Auto Supply.
 Uncover Support Options Tremfya Guselkumab
Uncover Support Options Tremfya Guselkumab
Tremfya Safe Returns Program.

Tremfya auto injector. Throw away See Step 3 after one dose even if there is medicine left in it. OTREXUP methotrexate soln pf auto-injector 225 mg04ml Brand 10119 Addition OTREXUP methotrexate soln pf auto-injector 25 mg04ml Brand 10119 Addition. Tremfya is administered as a 100 mg subcutaneous injection every eight weeks following two starter doses at weeks 0 and 4.
21 Plaque Psoriasis - TREMFYA is administered by subcutaneous injection. 3 DOSAGE FORMS AND STRENGTHS Injection. TREMFYA guselkumab soln prefilled syringe 100 mgml Brand 9119 Addition VALSTAR valrubicin soln for intravesical instillation 40 mgml Brand 10119 Removal generics.
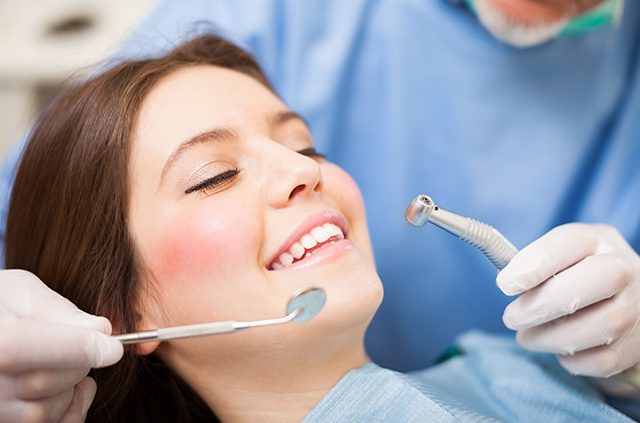
For adults with moderate to severe plaque psoriasis CLEARER SKIN THAT CAN LAST. TREMFYA comes in a single-dose One-Press injector containing one 100 mg dose. With an auto-injector the unit is placed against the skin and the needle automatically lowers after you press a button.
Made by JJ subsidiary Janssen Pharmaceuticals Horsham Penn Tremfya is a biologic therapy that selectively blocks only IL-23 a cytokine that plays a key role in plaque psoriasis according to Janssen. 1 single-dose One-Press patient-controlled injector 100 mg at Week 0 SHIP STARTER DOSE TO. The TREMFYA Injection Training Support Program is available for patients who have been prescribed TREMFYA by their dermatologist for either moderate to severe plaque psoriasis or active psoriatic arthritis.
The most common side effects of TREMFYA include. Do not reuse your One-Press injector. Toujeo insulin glargine injection Toujeo Sharps Disposal Program California residents only wwwsanofius.
TREMFYAis administered as a 100 mg subcutaneous injection once every 8 weeks after starter doses at weeks 0 and 4. Guselkumab is a fully human immunoglobulin G1 lamda IgG1λ monoclonal antibody mAb to the interleukin IL-23 protein produced in Chinese Hamster Ovary CHO cells by recombinant DNA technology. Engine 53L EcoTec3 V8 with Active Fuel Management Direct Injection and Variable Valve Timing includes aluminum block construction 355 hp 265 kW 5600 rpm 383 lb-ft of torque 518 N-m 4100 rpm Trans.
You may receive your first dose in the doctors office. In active psoriatic arthritis TREMFYAmay be administered alone or in combination with a cDMARD eg methotrexate. The injection is given in the upper arms thighs or abdomen at least 2 inches from your belly button.
These are not all the possible side effects of TREMFYA. Upper respiratory infections headache injection site reactions joint pain arthralgia diarrhea stomach flu gastroenteritis fungal skin infections herpes simplex infections and bronchitis. Each One-Press injector can only be used one time.
Tremfya is also used to treat active psoriatic arthritis in adults. TREMFYAis intended for use under the guidance and supervision of a physician. Prescriber office 1 single-dose prefilled syringe 100 mg at Week 0 Patient.
TREMFYA guselkumab Injection is a sterile preservative free clear colorless to light yellow solution that may contain small translucent particles. With only 6 maintenance doses of TREMFYA every 8 weeks after two starter doses at weeks 0 and 4. IL-23 is a naturally occurring cytokine that is involved in normal inflammatory and immune responses.
Tremfya 100 mg solution for injection in pre-filled pen Each pre-filled pen contains 100 mg of guselkumab in 1 mL solution. Prior Authorization is already on file with the patients plan for treatment with TREMFYA. The only difference is that a syringe has a plunger that you depress after the needle is inserted.
The injection procedure is essentially the same whether you are given a prefilled syringe or auto-injector. Tremfya is used to treat moderate to severe plaque psoriasis in adults. PRIOR AUTHORIZATION STARTER DOSE.
22 Psoriatic Arthritis - TREMFYA is. This medication is given by injection under your skin as directed by your doctor. Tremfya is a human monoclonal antibody that selectively binds to the p19 subunit of IL-23thereby inhibiting its interaction with the IL-23 receptor.
Tremfya guselkumab is a monoclonal antibody that blocks a certain protein in the body that can cause inflammation and other immune responses. 100 mgmL in a single-dose prefilled syringe or single-dose One-Press patient-controlled injector. This program provides patients with additional injection support to continue their TREMFYA treatment with confidence.
Individual results may vary. The recommended dose is 100 mg at Week 0 Week 4 and every 8 weeks thereafter. After the first dose another dose is usually given 4 weeks later followed by doses every 8 weeks.