How does fam-trastuzumab deruxtecan-nxki Enhertu work. ENHERTU fam-trastuzumab deruxtecan-nxki is a cytotoxic drug.
 Trastuzumab Deruxtecan Drug Description Adc Review
Trastuzumab Deruxtecan Drug Description Adc Review
Reconstitute and further dilute ENHERTU prior to intravenous infusion.

Trastuzumab deruxtecan package insert. Follow applicable special handling and disposal procedures. More than one vial may be needed for a full dose. ENHERTU fam-trastuzumab deruxtecan-nxki is a cytotoxic drug.
Fam-trastuzumab deruxtecan-nxki is compatible with an infusion bag made of polyvinylchloride or polyolefin copolymer of ethylene and polypropylene Administration 1. Calculate the dose mg the total volume of reconstituted. Pertuzumab intravenous trastuzumab and subcutaneous trastuzumab when administered alone.
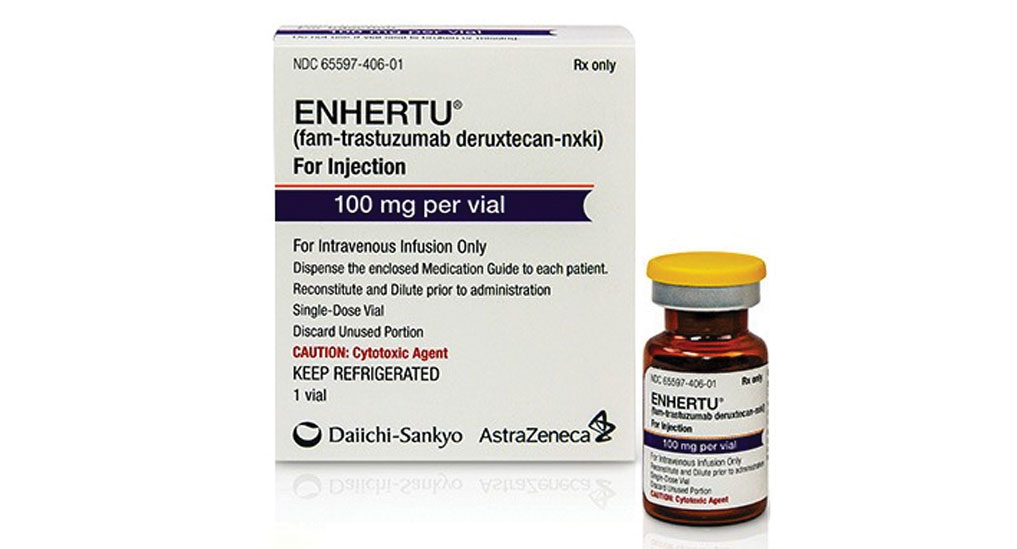
ENHERTU is a HER2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with. Enhertu 100 mg single-use vial. More than one vial may be needed for a full dose.
1 billable unit 1 mg NDC. ADCs are targeted cancer medicines that deliver cytotoxic chemotherapy payload to cancer cells via a linker attached to a. Follow applicable special handling and disposal procedures.
HER2-positive metastastic or advanced breast cancer who have received two or more treatment with anit-HER2-based regimens in the metastatic setting this indication is approved under accelerated approval based on response rate and. Referenced with permission from the NCCN Drugs Biologics Compendium NCCN. 1 billable unit 1 mg NDC.
ENHERTU fam-trastuzumab deruxtecan-nxki is a sterile white to yellowish white preservative-free lyophilized powder in single-dose vials. Enhertu 100 mg single-dose vial. Enhertu targets a protein on the surface of breast cancer cells known as Human Epidermal growth factor Receptor 2 HER-2.
HERCEPTIN Trastuzumab is a recombinant DNA-derived humanized monoclonal antibody that selectively binds with high affinity in a cell-based assay Kd 5 nM to the extracellular domain of the human epidermal growth factor receptor 2 protein HER2. Each vial delivers 100 mg of fam-trastuzumab deruxtecan-nxki L-histidine 445 mg L-histidine hydrochloride monohydrate 202 mg polysorbate 80 15 mg and sucrose 450 mg. Enhertu 100 mg single-dose vial.
ENHERTU en-HER-too is a prescription medicine used in adults to treat human epidermal growth factor receptor 2 HER2-positive. Once Enhertu binds to the breast cancer cell it enter the cell and releases a molecule called deruxtecan DXd a type of topoisomerase-I inhibitor designed to stop breast cancer cells from growing and dividing. Breast cancer that cannot be removed by surgery or that has spread to other parts of your body metastatic and who have received two or.
Use appropriate aseptic technique. Enhertu fam-trastuzumab deruxtecan-nxki in the US only. J9358 Injection fam-trastuzumab deruxtecan-nxki 1 mg.
Do not substitute PHESGO for or with pertuzumab trastuzumab ado-trastuzumab emtansine or fam-trastuzumab deruxtecan. Reconstitution Reconstitute immediately before dilution. J9358 Injection fam-trastuzumab deruxtecan-nxki 1 mg.
1 billable unit 1 mg NDC. Pronunciation of trastuzumab deruxtecan with 1 audio pronunciations 0 rating rating ratings Record the pronunciation of this word in your own. J9358 - Injection fam-trastuzumab deruxtecan-nxki 1 mg.
ENHERTU fam-trastuzumab deruxtecan-nxki is a cytotoxic drug. 12 The antibody is an IgG 1 kappa that contains human framework regions with the complementarity-determining regions of a. PHESGO must always be administered by a healthcare professional.
Enhertu is approved for the treatment of adults with. IV over 30 90 minutes with an infusion set made of polyolefin or polybutadiene and a 02- or 022-micron in-line polyethersulfone or polysulfone filter. Fam- trastuzumab deruxtecan DS-8201a is a novel antibody-drug conjugate composed of the anti-HER2 antibody and the.
Follow applicable special handling and disposal procedures1 Reconstitution Reconstitute immediately before dilution. How is Enhertu used. Unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the.
Therapies targeted to human epidermal growth factor receptor 2 HER2 have proven effective against tumors positive for HER2 amplification but there is an unmet clinical need for the treatment of tumors that express HER2 protein in the absence of HER2 amplification. ENHERTU fam-trastuzumab deruxtecan-nxki and not trastuzumab or ado-trastuzumab emtansine. Action Date Submission Supplement Categories or Approval Type Letters Reviews Labels Patient Package Insert Note Url.
Calculate the dose mg the. Trastuzumab deruxtecan outside the US is the lead product in the ADC Franchise of the Daiichi Sankyo Cancer Enterprise and the most advanced programme in AstraZenecas ADC scientific platform. This indication is approved under accelerated approval based on tumor.

 Enhertu Package Insert Public Health
Enhertu Package Insert Public Health
 Pdf Antitumor Activity And Safety Of Trastuzumab Deruxtecan In Patients With Her2 Low Expressing Advanced Breast Cancer Results From A Phase Ib Study
Pdf Antitumor Activity And Safety Of Trastuzumab Deruxtecan In Patients With Her2 Low Expressing Advanced Breast Cancer Results From A Phase Ib Study
 Enhertu Daiichi Sankyo Inc Fda Package Insert
Enhertu Daiichi Sankyo Inc Fda Package Insert
 Daiichi Sankyo Submits New Drug Application For Trastuzumab Deruxtecan To Japanese Regulators Adc Review
Daiichi Sankyo Submits New Drug Application For Trastuzumab Deruxtecan To Japanese Regulators Adc Review
 Targeting Her2 With Trastuzumab Deruxtecan A Dose Expansion Phase I Study In Multiple Advanced Solid Tumors Cancer Discovery
Targeting Her2 With Trastuzumab Deruxtecan A Dose Expansion Phase I Study In Multiple Advanced Solid Tumors Cancer Discovery
 Trastuzumab Deruxtecan Drug Description Adc Review
Trastuzumab Deruxtecan Drug Description Adc Review

 Trastuzumab Deruxtecan Drug Description Adc Review
Trastuzumab Deruxtecan Drug Description Adc Review
Https Www Modahealth Com Pdfs Med Criteria Enhertu Pdf
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2019 761139s000lbl Pdf
Https Www Nhpri Org Wp Content Uploads 2020 09 Um Onc 1379 Enhertu Fam Trastuzumab Deruxtecan Nxki 12282020 Pdf
 These Highlights Do Not Include All The Information Needed To Use Enhertu Safely And Effectively See Full Prescribing Information For Enhertu Enhertu Fam Trastuzumab Deruxtecan Nxki For Injection For Intravenous Use Initial U S Approval
These Highlights Do Not Include All The Information Needed To Use Enhertu Safely And Effectively See Full Prescribing Information For Enhertu Enhertu Fam Trastuzumab Deruxtecan Nxki For Injection For Intravenous Use Initial U S Approval
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.