Pazopanib is a multi-kinase inhibitor active against vascular endothelial growth factor receptors-1 -2 and -3 that is used in the therapy of advanced renal cell carcinoma and soft tissue sarcomas. FDA Approval for 2 other cancer types.
 Fda Approval For Votrient Pazopanib For Advanced Soft Tissue Sarcoma Article Nursingcenter
Fda Approval For Votrient Pazopanib For Advanced Soft Tissue Sarcoma Article Nursingcenter
Clinical Pharmacology Peginterferon Alfa-2b.
Pazopanib fda label. Pazopanib hydrochloride is a white to slightly yellow solid. See full prescribing information for complete boxed warning. It is very slightly soluble at pH 1 and practically insoluble above pH 4 in aqueous media.
Pazopanib 1 Oncology. FDAs approval was for pazopanib Votrient for the indication of renal cell carcinoma. Yes First approved October 19 2009 Brand name.
IFNL3 IL28B Clinical Pharmacology. NCCN and FDA-approved uses summarized for improved clarity STS. Each 200-mg tablet of VOTRIENT contains 200 mg of pazopanib equivalent to 2167 mg of pazopanib hydrochloride.
Votrient pazopanib is a kinase inhibitor indicated for the treatment of advanced renal cell carcinoma and advanced soft tissue sarcoma. Renal cell carcinomaa type of kidney cancer that is advanced. Ravi V et al 2016 Antitumor Response of VEGFR2- and VEGFR3-Amplified Angiosarcoma to Pazopanib J Natl Compr Canc Netw.
A reduced pazopanib dose of 200 mg once daily is recommended in patients with moderate hepatic impairment defined as an elevation of bilirubin 15 to 3 x ULN regardless of the ALT value see section 52. 400 mg tablets of VOTRIENT modified capsule-shaped yellow film-coated with. Severe and fatal hepatotoxicity has been observed in clinical trials.
References reviewed and updated. Approval which occurred on April 26 is based on a pivotal randomized double blind placebo controlled multicenter Phase 3 study known as PALETTE PAzopanib ExpLorEd in. Clinical Pharmacology Pazopanib 2 Oncology.
VOTRIENT tablets are for oral use. Because this drug is now approved for a different cancer it could be prescribed off-label for GIST at the discretion of a physician. Monitor hepatic function and interrupt reduce or discontinue dosing as recommended see Warnings and Precautions 51.
Grande E et al 2015 Pazopanib in pretreated advanced neuroendocrine tumors. Debossed on one side. In clinical trials with VOTRIENT hepatotoxicity manifested as increases in serum transaminases ALT AST and bilirubin was observed.
Each tablet contains 2167 mg of pazopanib hydrochloride equivalent to. Pazopanib hydrochloride is also being studied in the treatment of other types of cancer. 200 mg of pazopanib.
30 rijen Approval Date s and History Letters Labels Reviews for NDA 022465. Palliative therapy collapsed under the requirement for prior therapy. Pazopanib film-coated tablet 200 mg and 400 mg product-specific bioequivalence guidance PDF13256 KB Adopted.
It is used in patients who have already been treated with chemotherapy. The FDA has approved pazopanib Votrient GlaxoSmithKline for the treatment of patients with advanced soft tissue sarcoma who have previously received chemotherapy. Pazopanib hydrochloride is approved to treat adults with.
Pazopanib tablets for oral use Initial US. 51 Hepatic Toxicity and Hepatic Impairment. Each tablet contains 4334 mg of pazopanib hydrochloride.
A phase II open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors GETNE Ann Oncol. Each tablet contains 2167 mg of pazopanib hydrochloride equivalent to 200 mg of pazopanib. 5 WARNINGS AND PRECAUTIONS.
Renal Cell Carcinoma Soft Tissue Sarcoma. GS UHL debossed on one side. Specialist involvement in care and continuation of care statement added.
Equivalent to 400 mg of pazopanib. In October 2009 the FDA approved pazopanib Votrient for treatment of renal cell carcinoma. Pazopanib is not recommended in patients with severe hepatic impairment defined as total bilirubin.
The FDA reviewer noted that the applicant has already specified all the important risks as warnings and precautions in the proposed package label for pazopanib and further recommended a REMS that included the implementation of a Medication Guide and performing postmarketing pharmacoviligance. Debossed on one side. Soft tissue sarcomathat is advanced.
HIM new and Medicaid lines of business. Off-label uses added for uterine ovarian and thyroid cancer.
Fda Approves Gsk S Votrient For New Licence Pharmafile
 Trial Schema Randomized Phase Iii Trial Of Pazopanib In Locally Download Scientific Diagram
Trial Schema Randomized Phase Iii Trial Of Pazopanib In Locally Download Scientific Diagram
 Votrient Fda Prescribing Information Side Effects And Uses
Votrient Fda Prescribing Information Side Effects And Uses
 Pazopanib Pathway Pharmacokinetics
Pazopanib Pathway Pharmacokinetics
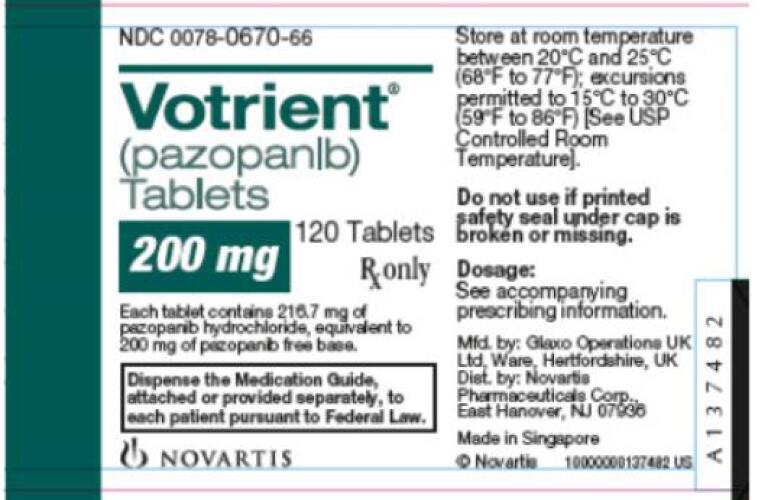
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2009 022465lbl Pdf
 Pazopanib Hydrochloride Wikidoc
Pazopanib Hydrochloride Wikidoc
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2012 022465s 010s 012lbl Pdf
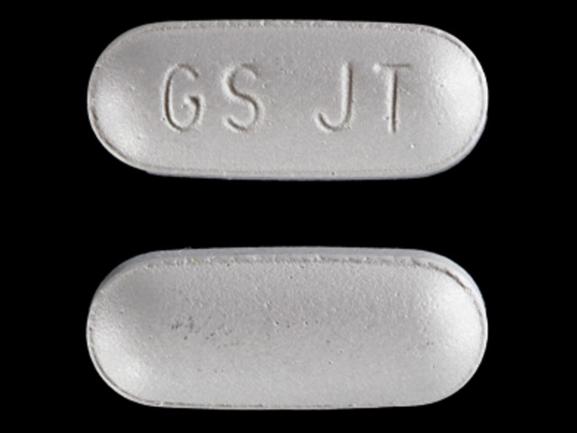 Votrient Fda Prescribing Information Side Effects And Uses
Votrient Fda Prescribing Information Side Effects And Uses
 Pazopanib Hydrochloride Wikidoc
Pazopanib Hydrochloride Wikidoc
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.